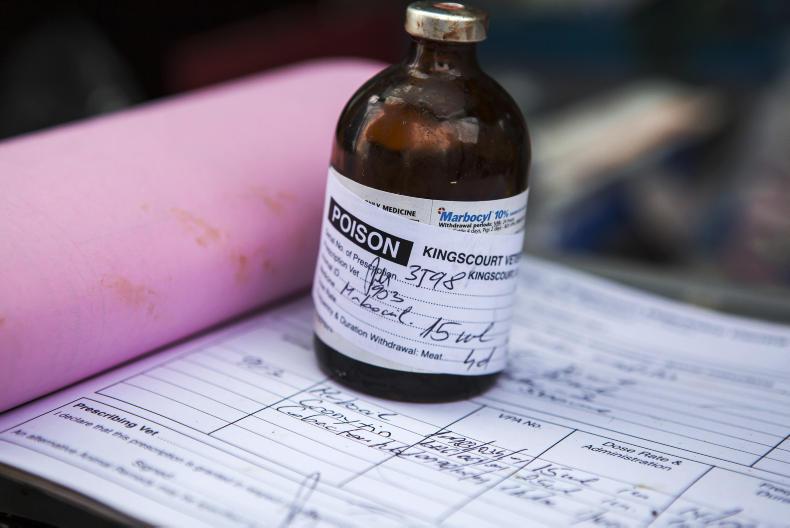
Tighter rules around the prescription of animal medicines and antibiotics have been adopted today (26 November) by the EU Commission.
The rules will be fully implemented by 2021 and are aimed at restricting the use of preventative blanket-use antibiotics through feed and other methods in a bid to prevent the risk of antimicrobial resistance (AMR).
The ban on using antibiotics for growth promotion which came into force in 2006 is upheld
Rules will include a higher level of charting the sale of antibiotics in member states and stricter control of animal imports from non-EU countries in regard to veterinary medicines.
Pragmatic implementation
The adoption of the legislation has been welcomed by Animalhealth Europe, which insists that a “pragmatic implementation” of the new legislation is needed.
“As is currently the case, the routine use of antibiotics to compensate poor hygiene, inadequate husbandry, care or management is not allowed, and the ban on using antibiotics for growth promotion which came into force in 2006 is upheld,” secretary general of AnimalhealthEurope Roxane Feller said.
“We support these measures and welcome additional rules to further improve responsible use of antibiotics in animal health.”
The rules which will come into force by 2021 include:
A ban on the preventive use of antibiotics in groups of animals.
- A ban on the preventive use of antimicrobials via medicated feed.
- Restrictions on metaphylactic use of antimicrobials (control treatment preventing a further spread of infection).
- A reinforced ban on the use of antimicrobials for promoting growth and increasing yield (in addition to the 2006 prohibition of using antibiotics as growth promoters in feed).
- The possibility to reserve certain antimicrobials for humans only.
- The obligation for member states to collect data on the sale and use of antimicrobials.
- Science-based maximum limits for cross contamination of feed with antimicrobials.
- Various measures aiming at prudent and responsible use of antimicrobials.
The EU Commission has yet to lay down detailed rules of how the tighter regulations will impact trade, but have said they should not be looked upon as a “trade barrier”, but rather as recognition that AMR does not respect borders.
Read more
UK milk processor introduces 0.5p/l herd health payment
Veterinary Council tightens prescription guidelines
Tighter rules around the prescription of animal medicines and antibiotics have been adopted today (26 November) by the EU Commission.
The rules will be fully implemented by 2021 and are aimed at restricting the use of preventative blanket-use antibiotics through feed and other methods in a bid to prevent the risk of antimicrobial resistance (AMR).
The ban on using antibiotics for growth promotion which came into force in 2006 is upheld
Rules will include a higher level of charting the sale of antibiotics in member states and stricter control of animal imports from non-EU countries in regard to veterinary medicines.
Pragmatic implementation
The adoption of the legislation has been welcomed by Animalhealth Europe, which insists that a “pragmatic implementation” of the new legislation is needed.
“As is currently the case, the routine use of antibiotics to compensate poor hygiene, inadequate husbandry, care or management is not allowed, and the ban on using antibiotics for growth promotion which came into force in 2006 is upheld,” secretary general of AnimalhealthEurope Roxane Feller said.
“We support these measures and welcome additional rules to further improve responsible use of antibiotics in animal health.”
The rules which will come into force by 2021 include:
A ban on the preventive use of antibiotics in groups of animals.
- A ban on the preventive use of antimicrobials via medicated feed.
- Restrictions on metaphylactic use of antimicrobials (control treatment preventing a further spread of infection).
- A reinforced ban on the use of antimicrobials for promoting growth and increasing yield (in addition to the 2006 prohibition of using antibiotics as growth promoters in feed).
- The possibility to reserve certain antimicrobials for humans only.
- The obligation for member states to collect data on the sale and use of antimicrobials.
- Science-based maximum limits for cross contamination of feed with antimicrobials.
- Various measures aiming at prudent and responsible use of antimicrobials.
The EU Commission has yet to lay down detailed rules of how the tighter regulations will impact trade, but have said they should not be looked upon as a “trade barrier”, but rather as recognition that AMR does not respect borders.
Read more
UK milk processor introduces 0.5p/l herd health payment
Veterinary Council tightens prescription guidelines








SHARING OPTIONS