It is estimated that nearly 80% of Irish dairy herds have at least one IBR-positive animal¹. These IBR carrier animals (latent carriers) are the source of the virus in IBR outbreaks. Although latent carriers can appear healthy within a herd, the underlying cost of IBR is significant. Research on Irish dairy farms has indicated that endemic IBR (as opposed to an outbreak of IBR) negatively impacts milk yield, with cows in uninfected herds yielding 250.9l per lactation more than cows in infected herds². Therefore, controlling IBR doesn’t only help to avoid unpredictable disease outbreaks, but is also an aid to maximise production.
‘IBR’ stands for infectious bovine rhinotracheitis’ and is caused by bovine herpes virus 1 (BoHV1).
Primary infection occurs when a naïve animal is exposed to the IBR virus through the nose or mouth following direct contact with an animal shedding the virus³, or indirectly via fomites or airborne transmission and is commonly associated with clinical signs of acute IBR disease which range from mild to severe. As IBR is caused by a herpes virus, not dissimilar to the human cold sore virus, it means that once an animal has been infected, it will become a latent carrier for life. Latent carriers are a potential source of infection and can shed virus at times of stress (transport, calving, nutritional stress) resulting in disease in the in-contact (naïve) animals³.
Clinical signs of IBR are variable ranging from subclinical disease to high body temperature, severe upper respiratory tract disease (the virus will target the eyes, nose and throat) and reproductive disease with symptoms of infertility and abortion4. In adult dairy cows sudden reduced milk production can be a feature of the disease.
How can you protect your herd from IBR?
Vaccination increases the overall immunity of the herd, and reduces shedding of virus from carrier animals, protecting the herd from a potential disease breakdown.
Live IBR vaccines stimulate superior cell mediated immunity and are best used in naïve animals (calves and weanlings) to protect them from clinical disease.
Live vaccines work rapidly and stimulate immunity most similarly to natural infection. This is because the virus is live (but altered to make it safe) so it can replicate in the body and therefore the body responds to it in a similar manner to the way it would to a natural infection. Studies have found that live vaccines are better at protecting naïve animals from clinical disease compared to inactivated ones5.
Inactivated IBR vaccines have been shown to stimulate a more robust humoral immunity or antibody response and are best used in latently infected animals (e.g. adult dairy cows) to stop or reduce viral shedding.
Inactivated vaccines stimulate immunity in a slightly different way to the live vaccines and have been found to be better at reducing viral shedding in animals which have previously been infected and are carrying the virus (latently infected carrier animals)6. In a dairy herd with a high bulk milk antibody level, which suggests that a high proportion of the milking cows are already infected, use of an inactivated vaccine can better help to reduce the amount of virus these cows shed when under stress. This reduces the risk to any uninfected animals within the herd, or heifers entering the herd.
The combination of a live and inactivated vaccine stimulates the immune-response in different ways resulting in a more complete immune response which provides 12-months protection from a single booster dose given once a year.
Join the tried and trusted programme
The Rispoval® Yearly IBR Programme has been tried and tested in Ireland since 2012, with both vets and farmers nationwide confident that they can rely on Rispoval for IBR protection.
A single dose of Rispoval IBR-Marker live in June/July will set up weanling heifers to join the Rispoval Yearly IBR programme this coming winter, when dairy farmers have more time to vaccinate the whole herd. With the Rispoval Yearly IBR programme animals over three months of age receive a single dose of Rispoval IBR Live (intramuscularly) followed by a single dose of Rispoval IBR Inactivated (under the skin) within six months. A yearly booster of Rispoval IBR- Marker inactivated is then required.
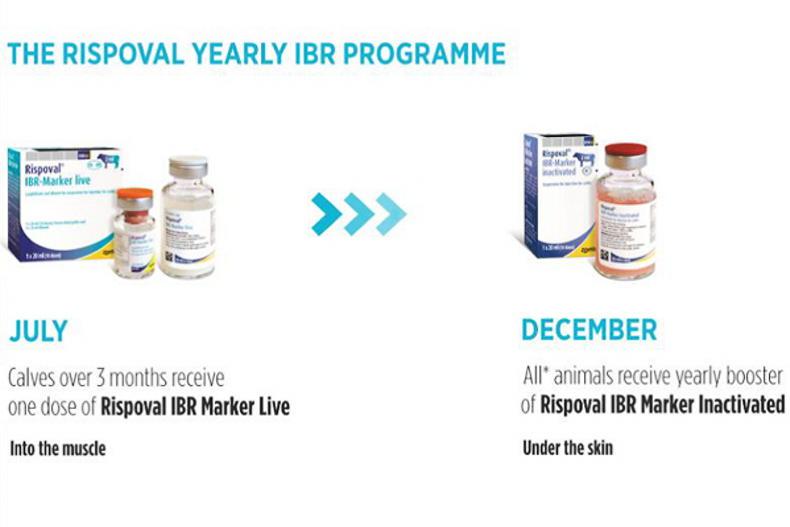
Rispoval Yearly IBR vaccination
Year 1: Cattle over three months of age receive one dose of Rispoval IBR-Marker live (intramuscularly) followed by one dose of Rispoval IBR-Marker inactivated within six months. This dose of Rispoval IBR-Marker inactivated provides one year IBR protection.
Year 2: A single yearly booster of Rispoval IBR-Marker inactivated should be given.
Advantages of the Rispoval Yearly IBR Programme
Lifetime herd approach. IBR protection for all animals from a young age. Reduction of virus shedding from existing latent carriers. Prevention of IBR abortion.** Labour saving as fewer vaccines required. Vaccination of the entire herd in December is both convenient and effective. Combining a live and an inactivated vaccine stimulates the immune response in different ways, resulting in a more complete immunity.No need to assess herd seroprevalence. It simply suits all herds.*All animals over three months must receive a single dose of Rispoval IBR Marker Live (within six months) before using Rispoval IBR Marker Inactivated on a yearly basis.
**In order to prevent abortion in female cattle that have only received their first single dose of Rispoval IBR-Marker live, the booster with Rispoval IBR-Marker Inactivated should be given no later than by the start of the second trimester of pregnancy.
References
1. Sayers, R. G., et al. (2015). Res. Vet. Sci.100:21–30.
2. Sayers R.G., (2017) J. Dairy Sci., Vol 100 (2): 1340-1352
3. Graham D., (2013), Irish Veterinary Journal, 66:15
4. Muylkens, B., et al., (2007), Veterinary Research 38: 181-209.
5. Bosch, J.C., et al. (1996), Vet. Microbiol., 52: 223-234.
6. Bosch J.C., et al. (1997). Vaccine 15:1512-1517
Rispoval® IBR-Marker live contains Bovine Herpes Virus type 1 (BHV-1), strain Difivac (gE-negative), modified live (attenuated) virus. Legal Status: POM(E)
Rispoval® IBR-Marker inactivated contains Bovine Herpes Virus type 1 (BoHV-1), strain Difivac (gE-negative) Legal Status: POM(E)
For further information please contact Zoetis, 2nd Floor, Building 10, Cherrywood Business Park, Loughlinstown, Co Dublin (01)2569800 or www.zoetis.ie
Use medicines responsibly see www.apha.ie ZT/21/21/1
It is estimated that nearly 80% of Irish dairy herds have at least one IBR-positive animal¹. These IBR carrier animals (latent carriers) are the source of the virus in IBR outbreaks. Although latent carriers can appear healthy within a herd, the underlying cost of IBR is significant. Research on Irish dairy farms has indicated that endemic IBR (as opposed to an outbreak of IBR) negatively impacts milk yield, with cows in uninfected herds yielding 250.9l per lactation more than cows in infected herds². Therefore, controlling IBR doesn’t only help to avoid unpredictable disease outbreaks, but is also an aid to maximise production.
‘IBR’ stands for infectious bovine rhinotracheitis’ and is caused by bovine herpes virus 1 (BoHV1).
Primary infection occurs when a naïve animal is exposed to the IBR virus through the nose or mouth following direct contact with an animal shedding the virus³, or indirectly via fomites or airborne transmission and is commonly associated with clinical signs of acute IBR disease which range from mild to severe. As IBR is caused by a herpes virus, not dissimilar to the human cold sore virus, it means that once an animal has been infected, it will become a latent carrier for life. Latent carriers are a potential source of infection and can shed virus at times of stress (transport, calving, nutritional stress) resulting in disease in the in-contact (naïve) animals³.
Clinical signs of IBR are variable ranging from subclinical disease to high body temperature, severe upper respiratory tract disease (the virus will target the eyes, nose and throat) and reproductive disease with symptoms of infertility and abortion4. In adult dairy cows sudden reduced milk production can be a feature of the disease.
How can you protect your herd from IBR?
Vaccination increases the overall immunity of the herd, and reduces shedding of virus from carrier animals, protecting the herd from a potential disease breakdown.
Live IBR vaccines stimulate superior cell mediated immunity and are best used in naïve animals (calves and weanlings) to protect them from clinical disease.
Live vaccines work rapidly and stimulate immunity most similarly to natural infection. This is because the virus is live (but altered to make it safe) so it can replicate in the body and therefore the body responds to it in a similar manner to the way it would to a natural infection. Studies have found that live vaccines are better at protecting naïve animals from clinical disease compared to inactivated ones5.
Inactivated IBR vaccines have been shown to stimulate a more robust humoral immunity or antibody response and are best used in latently infected animals (e.g. adult dairy cows) to stop or reduce viral shedding.
Inactivated vaccines stimulate immunity in a slightly different way to the live vaccines and have been found to be better at reducing viral shedding in animals which have previously been infected and are carrying the virus (latently infected carrier animals)6. In a dairy herd with a high bulk milk antibody level, which suggests that a high proportion of the milking cows are already infected, use of an inactivated vaccine can better help to reduce the amount of virus these cows shed when under stress. This reduces the risk to any uninfected animals within the herd, or heifers entering the herd.
The combination of a live and inactivated vaccine stimulates the immune-response in different ways resulting in a more complete immune response which provides 12-months protection from a single booster dose given once a year.
Join the tried and trusted programme
The Rispoval® Yearly IBR Programme has been tried and tested in Ireland since 2012, with both vets and farmers nationwide confident that they can rely on Rispoval for IBR protection.
A single dose of Rispoval IBR-Marker live in June/July will set up weanling heifers to join the Rispoval Yearly IBR programme this coming winter, when dairy farmers have more time to vaccinate the whole herd. With the Rispoval Yearly IBR programme animals over three months of age receive a single dose of Rispoval IBR Live (intramuscularly) followed by a single dose of Rispoval IBR Inactivated (under the skin) within six months. A yearly booster of Rispoval IBR- Marker inactivated is then required.

Rispoval Yearly IBR vaccination
Year 1: Cattle over three months of age receive one dose of Rispoval IBR-Marker live (intramuscularly) followed by one dose of Rispoval IBR-Marker inactivated within six months. This dose of Rispoval IBR-Marker inactivated provides one year IBR protection.
Year 2: A single yearly booster of Rispoval IBR-Marker inactivated should be given.
Advantages of the Rispoval Yearly IBR Programme
Lifetime herd approach. IBR protection for all animals from a young age. Reduction of virus shedding from existing latent carriers. Prevention of IBR abortion.** Labour saving as fewer vaccines required. Vaccination of the entire herd in December is both convenient and effective. Combining a live and an inactivated vaccine stimulates the immune response in different ways, resulting in a more complete immunity.No need to assess herd seroprevalence. It simply suits all herds.*All animals over three months must receive a single dose of Rispoval IBR Marker Live (within six months) before using Rispoval IBR Marker Inactivated on a yearly basis.
**In order to prevent abortion in female cattle that have only received their first single dose of Rispoval IBR-Marker live, the booster with Rispoval IBR-Marker Inactivated should be given no later than by the start of the second trimester of pregnancy.
References
1. Sayers, R. G., et al. (2015). Res. Vet. Sci.100:21–30.
2. Sayers R.G., (2017) J. Dairy Sci., Vol 100 (2): 1340-1352
3. Graham D., (2013), Irish Veterinary Journal, 66:15
4. Muylkens, B., et al., (2007), Veterinary Research 38: 181-209.
5. Bosch, J.C., et al. (1996), Vet. Microbiol., 52: 223-234.
6. Bosch J.C., et al. (1997). Vaccine 15:1512-1517
Rispoval® IBR-Marker live contains Bovine Herpes Virus type 1 (BHV-1), strain Difivac (gE-negative), modified live (attenuated) virus. Legal Status: POM(E)
Rispoval® IBR-Marker inactivated contains Bovine Herpes Virus type 1 (BoHV-1), strain Difivac (gE-negative) Legal Status: POM(E)
For further information please contact Zoetis, 2nd Floor, Building 10, Cherrywood Business Park, Loughlinstown, Co Dublin (01)2569800 or www.zoetis.ie
Use medicines responsibly see www.apha.ie ZT/21/21/1











SHARING OPTIONS