During 2018 herds participating in Phase Three of the Teagasc/Irish Farmers Journal BETTER Farm Beef Programme enrolled in the first phase of a Pilot IBR programme.
The programme was developed by Animal Health Ireland’s IBR Technical Working Group (TWG) in collaboration with Teagasc.
IBR (Infectious bovine rhinotracheitis) is a highly infectious disease caused by a virus called bovine herpes virus-1 (BoHV-1).
IBR has worldwide distribution and in addition to the impact on health and productivity also affects the trade of animals, semen and embryos.
In Ireland, BoHV-1 is mostly involved in respiratory infections, being one of the viral agents involved in the Bovine Respiratory Disease (BRD) complex. Infection with this virus is widespread in Ireland, with approximately 75% of beef and dairy herds containing animals that have been infected.
Cattle with IBR have a watery discharge from the nose and eyes and may present with red nose and eyes. Affected animals may be dull, off their feed and have a high temperature (107-108°F/41.7-42.5°C) and lack of appetite.
The severity of the clinical signs is influenced by a number of factors, including whether the animal has other infections, degree of stress and age.
During infection, the virus establishes latent infection in the nerve cells within the animal’s brain
Disease is typically milder in dairy herds, where milk drop can be a significant feature, and more severe in naive beef units.
Animals that survive infection recover but develop a latent or hidden infection, becoming lifelong carriers.
During infection, the virus establishes latent infection in the nerve cells within the animal’s brain.
During this latent period the carrier is not shedding virus.
However, at times of stress such as transport, calving, nutritional stress or mixing of stock, the virus may be reactivated and can begin to multiply and be re-excreted, generally from the nose and eyes.
This leads to new infections in other susceptible cattle, which in turn will also become latent carriers.
Airborne spread may also occur over distances of up to 5m
These latently infected carriers play a central role in maintaining IBR in infected herds, where they act as a reservoir of infection, and in spreading infection between herds.
The nasal discharge from infected animals can contain very high levels of virus and as a result infection can spread rapidly through a herd when susceptible cattle come in contact with infectious cattle or items contaminated by them such as feeders and drinkers.
It can also be shed from the reproductive tract, including semen, resulting in venereal transmission.
Airborne spread may also occur over distances of up to 5m.
Pilot programme
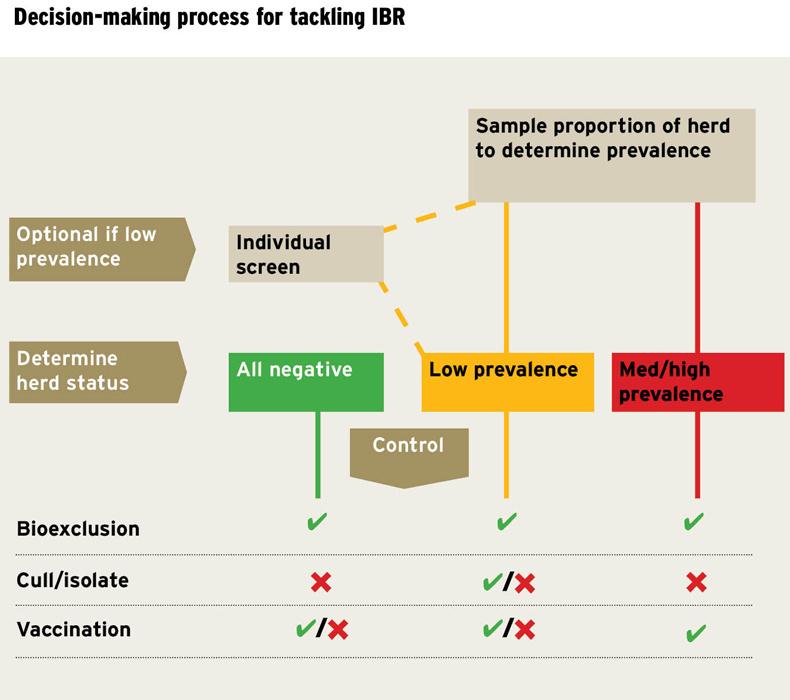
The pilot consisted in sampling and testing of a proportion of the herd for IBR, application of an IBR on-farm veterinary risk assessment and management plan (VIBRAMP), and provision of biosecurity and disease control advice.
BETTER Farms were initially screened by applying a herd snapshot, which requires the sampling of 30 randomly selected animals over nine months old that are used or intended for breeding.
This has been proposed as a cost-effective means to obtain an initial indication of the level of infection in a given herd.
If either no or only one animal is positive on the snapshot test, the proportion of infected animals within the herd (within herd prevalence) is estimated to be between 0% and 15%.
At this low prevalence, screening of the whole herd to identify and remove any carriers present is justified where herd freedom is the target.
If more than two seropositive animals are identified by the snapshot test, the likely within herd prevalence is greater than 15%.
In such herds, identification and removal of all carriers is unlikely to be feasible, and therefore other control measures are required until such times as a subsequent snapshot test indicates that sufficient progress has been made.
The VIBRAMP consists of a questionnaire carried out on farm by a trained private veterinary practitioner (PVP), that captures details of the farm structure, animal movements, biosecurity and vaccination history, with the vet and herdowner agreeing up to three changes to improve biosecurity.
Results to date
Fifty-nine per cent (17) of the herds had a negative (0 or 1 seropositive animals), with 11 herds having no positives and six having one.
Forty-one per cent (12) had a positive (two or more seropositive animals) snapshot test, of which three herds had two positives, eight had between three and seven seropositive animals and one herd had 14 positives.
Between 15 and 44 samples were submitted per herd, totalling to 909 samples.
A large proportion of the seropositive results were from older, non-homebred animals.
Analysis of results show that, on average, positive snapshot herds were larger than negative herds and had a higher number of animals introduced directly from other herds (moves from farm) than negative snapshot herds (Table 1).
The degree of expansion was calculated by dividing the average of births in the herds in 2017 with the average births in 2013/14. Positive herds experienced a higher degree of expansion (herds were 180% larger) than negative herds, which were 25% larger than in 2013/14.
Herds where stock are being introduced regularly (purchased, borrowed, contract-reared heifers, stock returning from shows and sales etc) are at a higher risk of introducing IBR as the biggest risk comes from animals of unknown health status. Neighbouring stock, visitors or equipment exposed to cattle on other farms also pose a significant risk.
What are the options now for these herds?
Negative snapshot herds
These herds are in a position to progress towards IBR freedom by testing the remainder of the herd and either confirming that all are negative or identifying and removing the small proportion of positive animals (See diagram above).
In addition, it will be very important to prevent IBR being re-introduced within these herds.
For this, a bioexclusion plan, including quarantine and testing of added animals, should be put in place in conjunction with their own PVP.
Reviewing the activities that might allow IBR to enter the herd and putting in place control measures for each one will be the main purpose of the plan.
Use of vaccination may be considered to protect the herd from infection but this is not necessarily the best option for all herds, especially for those where bioexclusion measures have been sufficient to date.
Positive snapshot herds
For these herds, particularly those with a high prevalence, it is not feasible to immediately progress to eradication by testing and removal of all seropositive animals.
Instead, it is recommended that a vaccination and biosecurity/bioexclusion plan be put in place to control the disease, leading to a reduction in the proportion of infected animals in the herd over time, until a negative snapshot is achieved.
The herd should be monitored regularly by testing a proportion to assess the success of the vaccination programme.
Complete and regular herd vaccination makes it less likely that a latent carrier will reactivate and shed the virus, and less likely that a naive animal will become infected and spread the virus after exposure.
However, the whole breeding herd and not just young stock must be vaccinated in order to achieve this effect.
Further analysis of the VIBRAMP findings is under way to identify any factors associated with herds being likely to test positive.
Discussions are planned in the coming weeks to allow each herd to decide on their next step.
Results from both the testing and the VIBRAMP from the BETTER Farms are being used to evaluate the herd status, to identify risk factors associated with the presence of infection, to identify common biosecurity risks and inform the decision on further testing and vaccination for the participating herds.
The information generated is being used by the IBR TWG to inform the development of options for a sustainable national IBR programme.
Mathematical modelling will support the evaluation of those options by allowing testing of different strategies and their effects on the success, duration and cost of such a programme.
This in turn will provide options for a consultation on progressing to a national programme.
Detailed information leaflets on IBR and herd biosecurity, along with answers to frequently asked questions on IBR and specific guidance for herds with bull calves that are potential AI sires, are available from AHI online.
The author would like to acknowledge all participating herd owners, Teagasc staff and PVPs for their contribution and Dr Liz Lane, DAFM, for the analysis of the results of this programme.










SHARING OPTIONS